CDMO
CDMO, contract development and manufacturing organization, is a form of outsourcing between industrial companies to provide required services for target industrial customers. CDMO in the diagnostic industry is mainly committed to providing IVD reagent/kit development and manufacturing services. The services provided include not only raw material selection, product research, development, stability research and testing, but also capacity expansion, commercialized production (finished products or intermediate products), etc. CDMO often has the dual capabilities of R&D and production, which is also a powerful manifestation of its capabilities.
As a leading professional CDMO company, DxPartner has established multiple technique application platforms for the research and manufacture of IVD kits/reagents for clinical diagnosis of different diseases. These platforms have been widely used and accepted by many customers, which have been successfully applied in the field of in vitro diagnosis. We can provide you with a customized one-stop CDMO service solution to meet your any special needs through leverage the rich experience in the IVD industry.
The Professional Process to Handle Customized CDMO Request from Customers
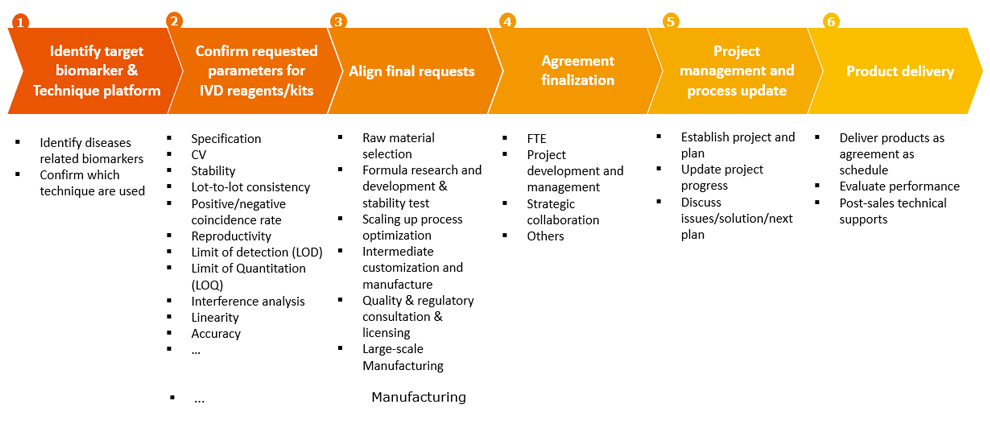
Meanwhile, DxPatner can provide customers with professional knowledge and experience by the requirements of local regulations/ policy and clinical applications of IVD products on product development, DxPartner also can provide expertise on product performance by different techniques and products to fit for the requirements of IVD end market, which can greatly reduce the cost of research and development, and improve the efficiency of research and development.
Pleasecontact us If you are interested in our services.
As a leading professional CDMO company, DxPartner has established multiple technique application platforms for the research and manufacture of IVD kits/reagents for clinical diagnosis of different diseases. These platforms have been widely used and accepted by many customers, which have been successfully applied in the field of in vitro diagnosis. We can provide you with a customized one-stop CDMO service solution to meet your any special needs through leverage the rich experience in the IVD industry.
The Professional Process to Handle Customized CDMO Request from Customers

Customization Requests
DxPartner has accumulated rich experience in product research and development to meet the specific needs of any IVD customers in new products and new platforms, including| ◆ Raw material screening and selection | ◆ New product development | ◆ Product performance test |
| ◆ Product production process optimization | ◆ Intermediate development and customization |
|
Large scale production service
DxPartner is owning a integrated production system for the production of QPCR, lateral flow and CLIA, and the professional production management system following strict regulatory requirement, which has served and many IVD companies worldwide.Meanwhile, DxPatner can provide customers with professional knowledge and experience by the requirements of local regulations/ policy and clinical applications of IVD products on product development, DxPartner also can provide expertise on product performance by different techniques and products to fit for the requirements of IVD end market, which can greatly reduce the cost of research and development, and improve the efficiency of research and development.
| Performance Technique | Lateral Flow |
QPCR |
CLIA |
Positive coincidence rate |
√ |
||
Negative coincidence rate |
√ |
||
Limit of Quantitation (LOQ) |
√ |
||
Accuracy |
√ |
√ |
|
Limit of detection (LOD) |
√ |
√ |
√ |
Lot-to-lot consistency |
√ |
√ |
√ |
CV |
√ |
√ |
√ |
Linearity |
√ |
√ |
|
Stability |
√ |
√ |
√ |
Sample Stability |
√ |
√ |
√ |
Specification |
√ |
√ |
√ |
Interference analysis |
√ |
√ |
√ |
Others |
√ |
√ |
√ |
Pleasecontact us If you are interested in our services.
